You know, sometimes the most foundational concepts in science are the ones that get the quickest brush-off in class. States of matter—solids, liquids, and gases—can sound pretty dry, right? But hold on a minute! That basic structure is the very foundation of chemistry and physics. It's literally the story of everything you and your students can see, touch, or breathe. You want to ignite that spark of curiosity, don't you? That's what Inspirational Science For Subs is all about—offering those innovative resources that go beyond the textbook and inspire a true love of learning. This isn't just about memorising definitions; it's about seeing the fundamental reality of the world.
Think about it: from the cup of tea you're holding to the air in the room, it's all about how those tiny particles behave. When you truly grasp the microscopic differences between a solid and a liquid, suddenly the melting of ice or the boiling of water isn't just a physical change; it's a profound molecular dance. You’ll be able to show your students that science isn’t just in the lab; it’s happening right in front of them every single moment. This blog post is packed with fun facts and ways to frame the states of matter that you might not know, which you can use to keep your students utterly engaged. Hopefully, this content will help you save time and inspire your students to see the magic in the everyday simple science of everything.
More...
Getting to Grips with the Three Main States of Matter
It’s often the case that students understand the three main states of matter—solids, liquids, and gases—at a surface level. They know a rock is a solid and steam is a gas. But do they truly grasp what's happening at the particle level? That's where the real teaching opportunity lies. The difference isn't about composition; it’s about energy and the arrangement of constituent particles. A block of ice and a puff of steam are both H2O molecules, but their energy levels are wildly different.
Solids: The Orderly Crew
In solids, the particles (atoms or molecules) are packed together tightly in a fixed, regular pattern. They don't move from place to place, they just vibrate around a fixed point. This is why a solid keeps its shape and volume, and why you can't easily compress it. Picture a crowded train carriage where everyone's standing shoulder-to-shoulder—they can only fidget, not walk around. That fixed arrangement makes for a low kinetic energy state. It’s simple, but sometimes you forget how important that vibrational motion is to define the state! This detail is a fantastic tie-in to thermal energy later on.
Liquids: The Free-Flowing Bunch
Now, add a little heat—give those particles some more kinetic energy. They gain enough energy to break free from their fixed positions but not enough to fly apart. They can slide past each other, which is why a liquid flows and takes the shape of its container. The volume stays relatively constant because they're still quite close together, but the loss of a fixed structure allows for that characteristic fluidity. Think of the train passengers now being able to mill about a bit, bumping into each other but still confined to the carriage. That ability to flow is the key difference when talking about the three main states of matter.
Gases: The High-Energy Rebels
Keep adding that energy and you get a gas. The particles are now moving very quickly and are far apart from one another. They have enough energy to completely overcome the forces of attraction between them, meaning they zoom around randomly in all directions. A gas doesn't have a fixed shape or volume; it will fill any container it's in, and you can easily compress it because there's so much empty space between the particles. This high kinetic energy explains why gases diffuse so rapidly.
- Question for the class: If you could magically swap the kinetic energy of the particles in a glass of water with the air around it, what immediate changes would you see in the water and the air?
Phase Changes: Switching Between the States of Matter
The beauty of the states of matter concept isn't just in defining the three forms, but in explaining how they transition from one to another. These phase changes (or state changes) are driven by the addition or removal of thermal energy. It’s the constant tug-of-war between the particles' kinetic energy and the forces holding them together. When you understand the movement of energy, suddenly all these phenomena make perfect sense. It’s what makes the simple science of everything so compelling!
Melting, Freezing, and Boiling
- Melting: Going from a solid to a liquid. You're adding heat, increasing the kinetic energy until the particles can no longer stay in their fixed positions. The temperature at which this happens is the melting point.
- Freezing: The opposite: liquid to solid. You’re removing heat (or thermal energy), allowing the particles’ reduced movement to let the forces of attraction lock them back into a rigid structure. This occurs at the freezing point, which is usually the same temperature as the melting point.
- Boiling: Liquid to gas. You're adding so much energy that the particles overcome all attractive forces and fly off completely. This happens throughout the liquid at the boiling point.
Evaporation, Condensation, and Sublimation
It’s important to stress that boiling isn't the only way to get a gas. Evaporation is a slower process, only happening at the surface of a liquid and occurring at temperatures below the boiling point. Only the highest-energy particles at the surface escape. Conversely, condensation (gas to liquid) happens when a gas cools down and loses energy, allowing attractive forces to pull the particles back into a liquid state.
But there’s a sneaky one: sublimation! This is when a solid turns directly into a gas, skipping the liquid phase entirely. Dry ice (solid carbon dioxide) is the classic example. It's a fantastic, dramatic visual to show your students just how versatile these states of matter can be. How cool is that?
- Question for the class: Imagine you have two identical puddles on a hot day. One is in a breezy, open area, and one is in a still, sheltered spot. Which one will evaporate faster, and how does the energy of the water molecules relate to that difference?
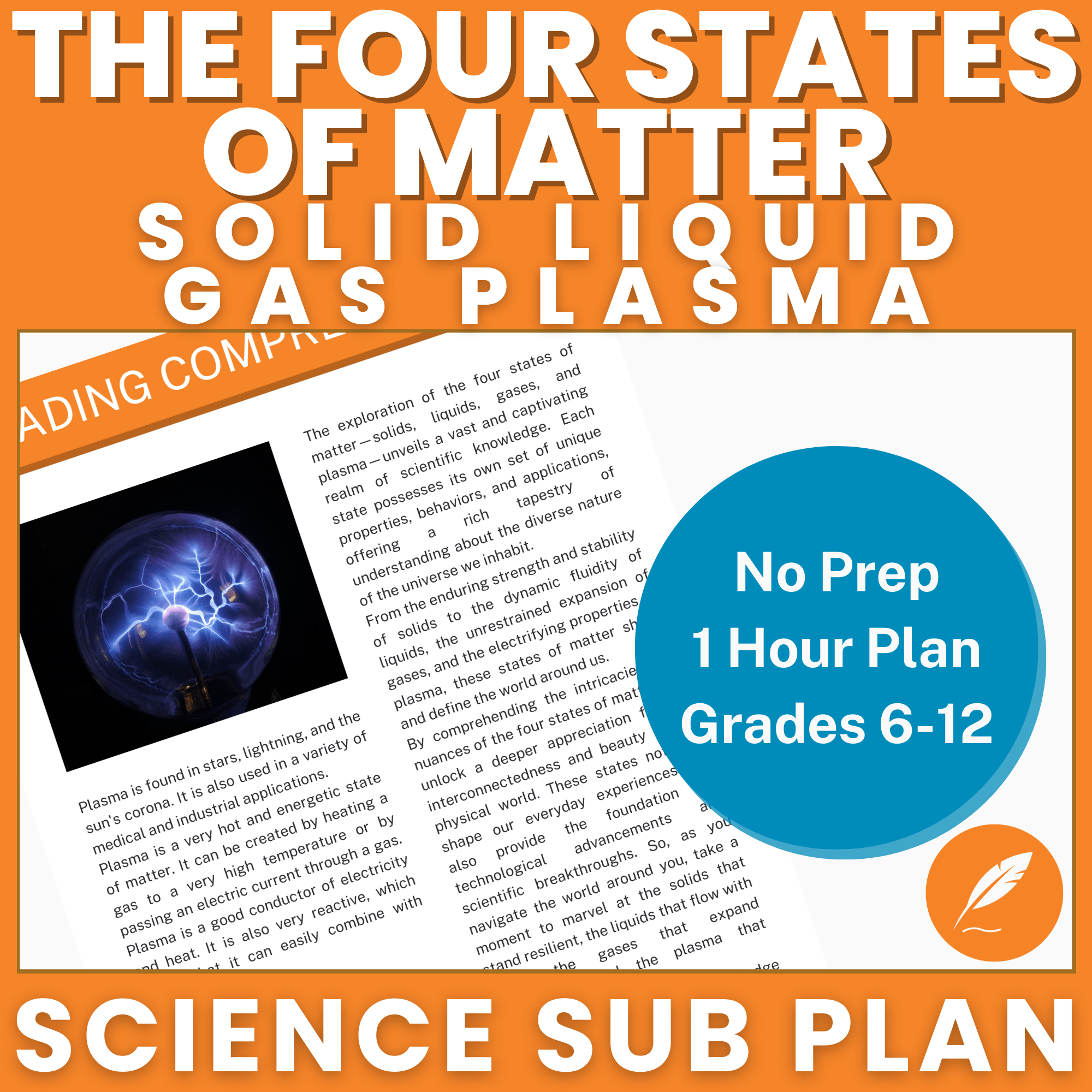
FREE Lesson Plan
Stop skimming the basics! This engaging, ready-to-use lesson plan for US Grades 6-12 makes teaching the States of Matter—including the amazing fourth state of matter, plasma—simple and fun.
Includes discussion questions to foster critical thinking and ignite a true love of learning.
Perfect for innovative subs and teachers who want to go Beyond the Textbook, Beyond Limits!
The Fourth State of Matter: Plasma
You've got solids, liquids, and gases, but to truly explain the simple science of everything, you need to talk about plasma. This is a concept that truly stretches the minds of students in grades 6-12 because it's not something you encounter often in everyday UK life—unless you’ve got a plasma TV, of course! Plasma is the most common state of normal matter in the universe, and it has some truly mind-boggling properties.
What Exactly Is Plasma?
Take a gas and heat it up a lot—we're talking thousands of degrees Celsius. The particles start moving so fast and colliding with such force that electrons are ripped away from the atoms. This creates a highly energetic soup of charged particles: ions (atoms that have lost or gained an electron) and free electrons. This electrically charged gas is plasma. Because it contains these charged particles, plasma conducts electricity and is heavily influenced by magnetic fields, which makes it incredibly useful in modern technology.
It’s often referred to as the fourth state of matter, and its properties are so distinct that it deserves its own category. Unlike a normal gas, it's highly conductive and glows when energy passes through it. The sun, the stars, and lightning are all examples of plasma in nature. Doesn't that make the lesson way more exciting? You're not just teaching about water and rocks; you're teaching about star stuff!
Plasma in Our World
- Natural Plasma: The sun, stars, lightning, and the gorgeous aurora borealis (Northern Lights). It’s evidence of the extreme temperatures and energy found in space.
- Artificial Plasma: Fluorescent light bulbs, neon signs, and, as mentioned, plasma display screens. The glowing light you see is the plasma releasing energy as electrons recombine with ions.
The fact that the vast majority of all known matter in the cosmos is in this fourth state of matter is a powerful way to inspire awe in your classroom. It elevates the topic from a simple observation of water to a discussion about the structure of the entire universe.
- Question for the class: If you could create a brand-new application for plasma that would benefit our town or city, what would it be and why would the plasma's ability to conduct electricity and glow be important?
Want engaged students? Explore how molecular energy dictates the states of matter—a foundational concept for all future science. @inspirationalscienceforsubs #Physics #ChemEd
Beyond the Basics: Unusual Examples of States of Matter
Once your students have a solid understanding of the four main states of matter, you can really push their critical thinking by introducing some of the more exotic states. This is the sort of content that separates the curious from the completely fascinated, and it’s a brilliant way to encourage exploration and a deeper love of learning. It shows them that science is an ongoing process with more questions than answers!
The Bose-Einstein Condensate (BEC)
This one is wild. Take a gas and cool it down to temperatures incredibly close to absolute zero (the coldest possible temperature, about -273.15°C). The atoms virtually stop moving and start behaving like one single "super-atom" or wave rather than individual particles. This is the Bose-Einstein Condensate, or BEC. It’s an incredibly low-energy state where the quantum properties of matter become apparent on a macroscopic scale. It’s the ultimate opposite of plasma. It's a fantastic real-world example of how particle energy dictates the behaviour of the states of matter.
Liquid Crystals
If you've got a digital watch or a calculator, you've got liquid crystals. They’re not technically a fifth state, but they have properties of both a liquid and a solid. Their molecules flow like a liquid, yet they maintain a degree of ordered structure (like a crystal solid) in certain orientations. They can be manipulated by an electric field to block or pass light, which is how LCD screens work. It's a brilliant example of a transitional state and further shows that the lines between the primary states of matter can be blurry.
Using these extreme or unusual examples helps reinforce the core lesson: the defining factor for any state is the energy and arrangement of its constituent particles. The simple science of everything suddenly becomes much more complex and rewarding to explore!
- Question for the class: If you could travel to a planet where the average temperature was near absolute zero, how would the properties of the common elements there (like oxygen or nitrogen) be different from what we experience on Earth, and which unusual state of matter might you observe?

Enjoyed the article?
Inspiring Your Students with the Simple Science of Everything
Teaching the states of matter is a golden opportunity to make physics and chemistry feel relevant and exciting. You're not just teaching about science; you're teaching how the universe works at its most fundamental level. The energy of a particle dictates whether it's part of a rock, a puddle, or a lightning bolt. That's powerful stuff!
The key is linking the abstract particle level to tangible, everyday examples. When discussing gases, talk about the immense pressure in a scuba tank and how the gas particles are being forced close together. When talking about plasma, discuss the cutting-edge fusion reactors scientists are trying to build. You won't have to waste time re-explaining the same thing year after year if you establish this solid foundation of the three—and four!—main states of matter early on.
It truly is the simple science of everything, and taking the time to explore the extremes, like the BEC or plasma, encourages the kind of critical thinking and problem-solving that Inspirational Science For Subs aims to foster. Trust me, spending a little extra time on the seemingly simple concept of the states of matter makes a big difference in how well your students grasp all future science concepts. They'll appreciate the depth, and you'll love the engagement!
- Question for the class: Imagine you’re a sci-fi writer. What completely fictional fifth state of matter could you invent that has properties unlike anything we’ve discussed, and what would its unique particle energy and arrangement be?
Summary: Mastering the States of Matter for Inspired Learning
You’ve got so much to cover in the curriculum, but taking the time to really nail the basics of the states of matter will pay dividends for your students. We've gone over the three main forms—solids, liquids, and gases—and how they differ purely by the kinetic energy and arrangement of their particles. You've now got great facts and discussion points about phase changes, including the less common sublimation. Don’t forget to introduce the amazing, charged world of plasma, the fourth state of matter, which makes up the sun and stars!
It's all about making science feel real and relevant. By presenting these concepts with energy and using unusual examples like liquid crystals or the BEC, you're encouraging exploration and showing that the simple science of everything isn't so simple after all—it’s fascinating! Your students are going to feel inspired when they realise they’re learning about the fundamental building blocks of the entire universe. What's your favourite, most unexpected demonstration you use to illustrate the difference between the states of matter in your classroom? Let me know in the comments below!
